From Lab to Life: Exploring the Mystery of HCOOCH CH₂ H₂O
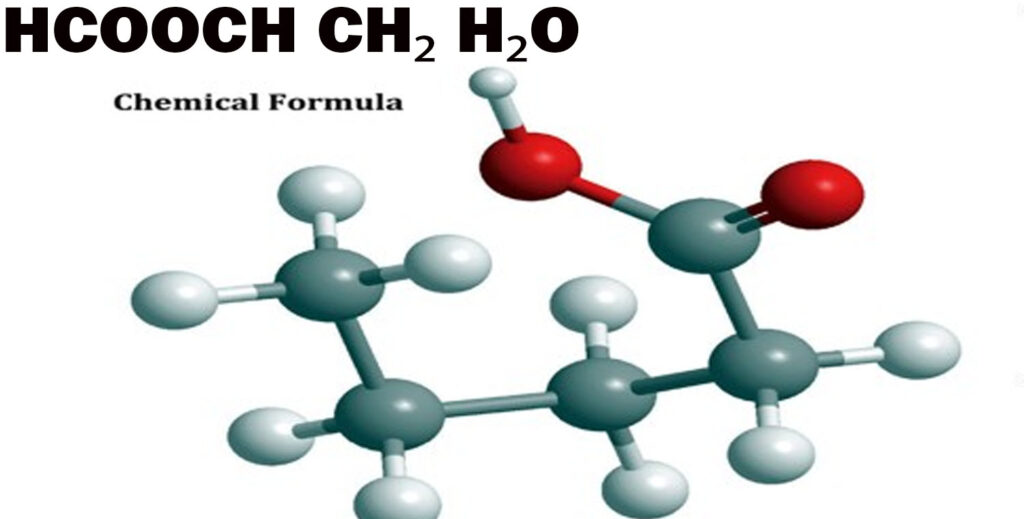
What is HCOOCH CH₂ H₂O?
HCOOCH CH₂ H₂O appears as a composite chemical notation, where the central component is methyl formate (HCOOCH₃). The inclusion of CH₂ and H₂O suggests either a reaction pathway or a reaction mixture, possibly involving hydrolysis or an intermediate process in organic synthesis. While not a single standard compound, this notation is commonly used to describe systems where methyl formate interacts with water and methylene groups, offering insight into reactivity and structural dynamics.
Common Misconceptions and Confusion with Similar Compounds
Many students and even professionals misinterpret HCOOCH CH₂ H₂O as a single compound. In reality, it may reference reactants or intermediates in a larger chemical process. It is often mistaken for methyl acetate or methanol-based esters, but their structure and behavior differ significantly. Clarifying this helps avoid errors in both industrial applications and academic research.
Chemical Nomenclature and IUPAC Classification
IUPAC Naming of HCOOCH
The core molecule, HCOOCH₃, is formally known as methyl methanoate under IUPAC nomenclature. It consists of a formate group (HCOO–) attached to a methyl group (–CH₃), categorizing it as an ester derived from formic acid and methanol.
Understanding CH₂ and H₂O’s Roles
The CH₂ unit typically represents a methylene bridge in organic compounds, often used in polymer backbones or hydrocarbon chains. Meanwhile, H₂O (water) can act as a solvent, product (during esterification), or a reactant (in hydrolysis). In the context of HCOOCH CH₂ H₂O, water is most likely a participant in the hydrolysis of methyl formate, forming formic acid and methanol.
Dissecting the Structure
The Molecular Formula and Atomic Composition
The molecular formula for methyl formate (HCOOCH₃) is C₂H₄O₂, comprising:
- 2 carbon atoms
- 4 hydrogen atoms
- 2 oxygen atoms
When CH₂ and H₂O are added as separate entities or intermediates, the total molecular profile depends on the reaction context.
Functional Groups Present
- Ester group (–COO–): Responsible for the reactivity in hydrolysis and esterification.
- Methyl group (–CH₃): Adds nonpolarity and volatility.
- Methylene group (–CH₂–): Common in hydrocarbon chains and bridge molecules.
- Water (H₂O): A polar molecule with significant reactivity potential in organic chemistry.
Electron Configuration and Bonding
Methyl formate’s structure includes polar covalent bonds, especially around the carbonyl carbon (C=O), which is susceptible to nucleophilic attack. The ester linkage forms a resonance-stabilized structure, and water, when involved, can attack this linkage, leading to hydrolysis.
Structural Breakdown
HCOOCH – Methyl Formate
This ester is colorless, volatile, and has a pleasant smell. It is widely used in chemical syntheses due to its reactive ester group and its ability to act as an intermediate.
CH₂ – The Methylene Bridge
The methylene (CH₂) unit serves as a connecting bridge in organic molecules. It is often involved in reactions that extend carbon chains or introduce side groups.
H₂O – Water as Solvent or Product
In many organic reactions, water can either act as the medium for the reaction or as a byproduct of condensation reactions. In hydrolysis, it is a critical reactant breaking down esters like methyl formate.
Physical Properties
Molecular Weight
The molecular weight of methyl formate is approximately 60.05 g/mol, making it a low molecular mass compound ideal for rapid evaporation and transport.
Boiling and Melting Points
- Boiling point: ~31.5°C (very low, leading to high volatility)
- Melting point: −100°C
These properties make methyl formate highly suitable as a solvent and for use in quick-drying applications.
Solubility in Various Solvents
Methyl formate is:
- Soluble in ethanol, ether, and acetone
- Moderately soluble in water, which affects how it interacts during hydrolysis and extraction.
Chemical Properties
Reactivity
Methyl formate readily undergoes hydrolysis in the presence of acids or bases, yielding formic acid and methanol. It also reacts with Grignard reagents and can participate in transesterification reactions.
Combustibility
It is highly flammable, forming explosive vapors when mixed with air. It requires careful handling in environments where heat or sparks may occur.
Stability Under Different Conditions
Methyl formate is relatively stable under cool, dark, and dry conditions, but can decompose under intense heat or in the presence of strong acids or bases.
Synthesis and Production
Industrial Synthesis Methods
In industry, methyl formate is produced via carbonylation of methanol: CH₃OH + CO→HCOOCH₃\text{CH₃OH + CO} \rightarrow \text{HCOOCH₃}CH₃OH + CO→HCOOCH₃
This reaction uses sodium methoxide as a catalyst and operates under controlled temperature and pressure.
Laboratory Preparation
In labs, it is synthesized by reacting formic acid with methanol in the presence of sulfuric acid as a catalyst. This Fischer esterification method also yields water as a byproduct.
Safety Measures During Synthesis
Always operate in well-ventilated areas, use flame-proof equipment, and wear protective gloves and eyewear. Emergency measures should be in place for leaks or exposure.
Applications in Chemistry
Intermediate in Organic Reactions
Methyl formate acts as a precursor in the synthesis of formamides, formates, and various pharmaceutical intermediates. It is often used in reduction reactions and carbonylation pathways.
Role in Esterification and Hydrolysis
In esterification, methyl formate can serve as both a starting material and a product, and its hydrolysis is commonly studied to understand ester breakdown mechanisms.
Applications in Industry
Use in Perfumes and Fragrances
Due to its fruity aroma, methyl formate is incorporated in flavor and fragrance formulations, particularly in floral and fruity notes.
Solvent Uses
Its fast evaporation and low boiling point make it ideal in paint removers, adhesives, and cleaning agents.
Role in Pharmaceuticals
It serves as an intermediate in synthesizing drugs and APIs (active pharmaceutical ingredients), especially where formylation steps are required.
Environmental Impact
Biodegradability
Methyl formate is biodegradable, breaking down naturally in the presence of microbes, light, and air.
Potential Hazards and Regulations
It is regulated under various environmental and safety bodies like OSHA and EPA due to its flammability and potential health effects.
Handling Spillages and Disposal
Small spills should be absorbed with inert material. Dispose of in accordance with local environmental regulations. Never pour it down the drain or into open soil.
Biological Interactions
Toxicity to Humans and Animals
High exposure can result in respiratory irritation, drowsiness, and organ toxicity. Inhalation of concentrated vapors or ingestion is dangerous.
Metabolism in the Body
In the human body, methyl formate is broken down into methanol and formic acid, which can affect the central nervous system and cause metabolic acidosis in large quantities.
Storage and Handling Guidelines
Safe Storage Conditions
Store in cool, well-ventilated areas, away from heat and ignition sources. Containers should be tightly sealed and labeled clearly.
Precautionary Measures
Wear chemical-resistant gloves, eye protection, and use explosion-proof equipment in storage and handling zones. Install gas detection systems in areas with high usage.
Comparison with Similar Compounds
Methyl Acetate vs. Methyl Formate
While both are esters, methyl acetate (CH₃COOCH₃) has a higher boiling point and different odor profile. It is less reactive toward hydrolysis compared to methyl formate.
Distinction Between Esters and Other Functional Groups
Esters (–COOR) are easily distinguished from ketones (–C=O), ethers (–O–), and alcohols (–OH) by their unique carbonyl-oxygen bonding pattern, influencing their reactivity and polarity.
Future Trends and Research
New Derivatives Under Study
Researchers are exploring substituted methyl formates for higher selectivity, better thermal stability, and use in pharmaceutical synthesis.
Green Chemistry Approaches for Production
There is a growing push toward using bio-based methanol and eco-friendly catalysts to reduce the carbon footprint of methyl formate production.
Conclusion
Summary of Key Points
HCOOCH CH₂ H₂O represents more than a mere chemical mixture—it opens the door to understanding ester chemistry, hydrolysis, and industrial applications. With methyl formate at its core, its behavior in reactions and roles in various industries make it indispensable.
Practical Outlook
As research advances, the shift toward safer production and green alternatives makes methyl formate not just useful but also sustainable. Its diverse applications ensure it will remain relevant across chemistry, pharmaceuticals, and environmental science.
FAQs about hcooch ch2 h2o
What is FAQsused for?
It’s primarily used as a solvent, intermediate in organic synthesis, and a fragrance additive.
Is HCOOCH CH₂ H₂O safe?
In controlled environments, yes. However, it is flammable and toxic in high concentrations, requiring careful handling.
Can you drink methyl formate?
Absolutely not. It is toxic and may lead to severe health complications or death if ingested.
What happens when HCOOCH reacts with water?
It undergoes hydrolysis, producing formic acid and methanol, especially under acidic or basic conditions.
How is HCOOCH produced industrially?
By reacting carbon monoxide with methanol in the presence of a base like sodium methoxide, under controlled conditions.






Responses